The world's largest repository of adverse effects from medicines, including COVID-19 "vaccines," is the collection of individual case safety reports (ICSRs) maintained by the World Health Organization (WHO). Created in 1968, the WHO Programme for International Drug Monitoring is a group of more than 150 member countries that work nationally and collaborate internationally to "identify and monitor the harm caused by medicines, to reduce the risks to patients and to establish worldwide pharmacovigilance standards and systems." Called VigiBase, the collection has over 25 million reports of suspected adverse effects of medicines. The WHO describes the project as:
"A global database containing reports of suspected side effects of medicines, also known as adverse drug reactions. Where a side effect is suspected of being related to a certain medicine or vaccine."
 Members of the WHO Programme for International Drug Monitoring. Dark blue: Full member. Light blue: Associate member. White: Non-member. For more information about individual members of the WHO programme, click here.
Members of the WHO Programme for International Drug Monitoring. Dark blue: Full member. Light blue: Associate member. White: Non-member. For more information about individual members of the WHO programme, click here.
 Exhibit 1. An October 5, 2021 search for "COVID-19 vaccine" at www.vigiaccess.org, the WHO/UMC public access website to overview statistics from VigiBase. Total number of records retrieved: 2,183,912.
Exhibit 1. An October 5, 2021 search for "COVID-19 vaccine" at www.vigiaccess.org, the WHO/UMC public access website to overview statistics from VigiBase. Total number of records retrieved: 2,183,912.
The Drug Monitoring Program of the WHO and UMC
The WHO Programme for International Drug Monitoring—established after the thalidomide disaster of 1961—is developed and maintained by Uppsala Monitoring Centre (UMC), which has managed the data since 1978. The proposal for the WHO program came in 1962 at the World Health Assembly following outrage over horrific abnormalities in children born to mothers who took thalidomide. Severe birth defects were seen in children born to mothers who had been prescribed the drug during pregnancy, and these were found to be connected with thalidomide.
In a devastating outcome, more than 10,000 cases of birth defects resulting from thalidomide were reported in over 46 nations. They included children born with missing or irregular limbs, spinal cord defects, cleft lip or palate, absent or abnormal ears, heart, kidney, genital abnormalities, and abnormal formation of the digestive system. Nearly 40 percent of the thalidomide child victims died within a year of birth. In 1961, many countries removed the drug from the market. The WHO-UMC website describes the tragedy:
"The discovery that severely malformed babies were being born to mothers who had taken a supposedly "safe" sleeping pill during pregnancy abruptly ended an age of uncritical trust in medicines. It caused worldwide outrage. In 1962, a proposal was made at the World Health Assembly that an international system for monitoring the adverse effects of medicines, based on reports from national agencies, should be established to prevent such a tragedy [from] ever happening again."
 Oct. 5, 2021 VigiAccess Breakout of "General disorders and administration site conditions" as shown in Exhibit 1 search results for COVID-19 Vaccines; 1,333,876 events
Oct. 5, 2021 VigiAccess Breakout of "General disorders and administration site conditions" as shown in Exhibit 1 search results for COVID-19 Vaccines; 1,333,876 events
What is Pharmacovigilance?
The WHO defines pharmacovigilance (PV) as "the science and activities relating to the detection, assessment, understanding, and prevention of the adverse effects or any other possible drug-related problems." The primary focus of the PV practice (initiated soon after the thalidomide disaster) has been centered around collecting, assessing, and reporting adverse drug reactions to medicinal products. A 2018 NIH abstract titled "Comparative evaluation of pharmacovigilance regulation of the United States, United Kingdom, Canada, and India and the need for global harmonized practices" reminds the reason for the creation of the WHO program:
"The WHO International Programme for adverse reaction monitoring led to the identification of the rare adverse drug reactions (ADRs) that could not be identified through the limited scope of clinical trials."
 Oct. 5, 2021 VigiAccess Date related to Geographical distribution as shown in Exhibit 1 search results for COVID-19 Vaccines.
Oct. 5, 2021 VigiAccess Date related to Geographical distribution as shown in Exhibit 1 search results for COVID-19 Vaccines.
What is VigiBase?
Nearly half of the VigiBase data is from the United States, and close to 20 percent is from the European Union. Other countries, like Asia, are rapidly increasing the amount of information they share, and the proportion of data coming from low- and middle-income countries is expanding. The VigiBase database system is linked to medical and drug classifications, including specifications such as MedDRA, and the medicinal products dictionary, WHODrug.
The VigiBase system depends on participating nations for "the timeliness, completeness, and quality of reports [which] relies to a large extent on the goodwill of the participating pharmacovigilance centres." Additionally, since the level of suspicion required for making a report varies from country to country, the reports in the system are of a "suspected side effect," instead of a "confirmed side effect." The database points out that a "search of VigiBase will not reveal the safety profile of any vaccine or medicine." Still, it flags the adverse reaction as something that "needs to be looked into further."
VigiBase is available to the members of the WHO Programme for International Drug Monitoring. Individuals with a degree in one of the health professions can also request to be granted access. The general public can access summary statistics from VigiBase through the VigiAccess website—a search interface that allows visitors to access statistics on reported adverse reactions to medicines and vaccines.
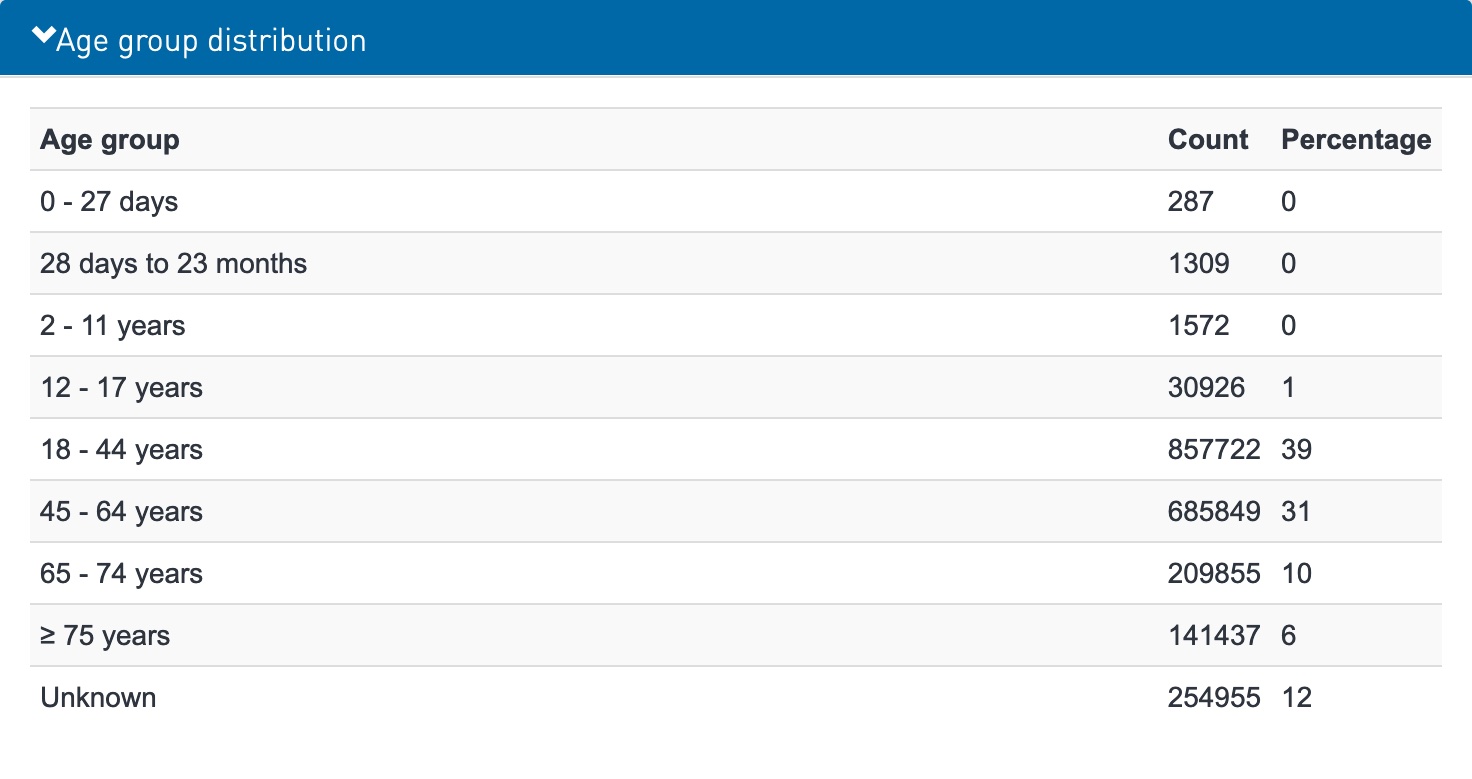

 Oct. 5, 2021 VigiAccess data related to Age group distribution; ADR reports per year; and Patient sex distribution, as shown in Exhibit 1 search results for COVID-19 Vaccines.
Oct. 5, 2021 VigiAccess data related to Age group distribution; ADR reports per year; and Patient sex distribution, as shown in Exhibit 1 search results for COVID-19 Vaccines.
Reporting of Adverse Event Data in the U.S.
In the United States, the Vaccine Adverse Event Reporting System (VAERS) relies on individuals to report their experiences. Anyone can submit data to the passive VAERS reporting system, including parents and patients. It is well documented that the numbers in VAERS represent a tiny percentage of actual adverse vaccine-related events. Likewise, the FDA's Adverse Events Reporting System (FAERS) relies on reports voluntarily submitted by parents, consumers and healthcare professionals directly to the FDA through the MedWatch program. Like VAERS, the data on FAERS is unreliable, with "many instances of duplicative reports and some reports do not contain all the necessary information."
 JMIR Public Health and Surveillance, NIH, Published online 2018 Feb 27. doi: 10.2196/publichealth.9282
JMIR Public Health and Surveillance, NIH, Published online 2018 Feb 27. doi: 10.2196/publichealth.9282
Following the CDC's cessation in May 2021 of full reporting on COVID-19 hospitalizations and deaths, the agency switched gears to focus on its preferred "COVID-19 Vaccine Reporting Systems." Still, they appear to lack comprehensive or readily available accurate data on adverse events associated with the current COVID-19 EUA "vaccines" increasingly mandated by the Biden administration for every human being in the nation.
A 2018 NIH report titled, "Why Clinicians Don't Report Adverse Drug Events Qualitative Study" concluded:
"While providers routinely document adverse drug events in clinical records to inform patient care, barriers exist to report to external agencies. Existing reporting systems are not suited to capture the complex nature of adverse drug events or adapted to workflow and are simply not used by frontline clinicians."
 Oct. 5, 2021, VigiAccess Breakout of "Reproductive system and breast disorders" as shown in Exhibit 1 search results for COVID-19 Vaccines; 84,169 events.
Oct. 5, 2021, VigiAccess Breakout of "Reproductive system and breast disorders" as shown in Exhibit 1 search results for COVID-19 Vaccines; 84,169 events.
UMC, WHO and VigiBase Data
The operations of UMC as a WHO Collaborating Centre are governed by an agreement between WHO Headquarters and the Swedish government. UMC has an international board. In 2018, the United States, as the largest donor, gave a voluntary contribution of nearly $300 million of its over $2.3 billion in donations. The mission of UMC is "to support and promote patient safety through effective and global pharmacovigilance practice." Their website states they achieve this by:
- Providing independent and high-quality medical reference sources globally.
- Exploring the risks and benefits of medicines, advancing the science of pharmacovigilance.
- Transforming medicine safety data into best practice.
- Building global pharmacovigilance capacity.
- Encouraging local and regional pharmacovigilance initiatives by opening access to adequate resources and providing tailor-made guidance.
- Building partnerships that bring together resources, expertise, and a shared vision.
- Turning knowledge into action by enabling stakeholders to identify issues, find answers, and drive change.
- Pursuing technological change, developing communications capacity and ensuring that our sustainability is underpinned by secure funding.
Finally, the partners declare their vision "is a world where all patients and health professionals make wise therapeutic decisions in their use of medicines." Nonetheless, with the unprecedented massive campaign underway to mandate COVID-19 "vaccines" for every man, woman, and child walking the earth, it is not clear how their vision advocates for those who make the personal decision to not receive the jab.


