The "Risk Factors" section of Moderna's Form 10-K filing for the fiscal year ending December 31, 2021, is alarming for anyone who received the pharmaceutical giant's mRNA gene-therapy jab. Filed with the U.S. Securities and Exchange Commission (SEC) to inform current shareholders or potential investors about Moderna's performance, the 37-page section of the 160-page filing discloses the uncertainty surrounding the company's COVID-19 "investigational medicines" and mRNA technology in general—which the company calls "the software of life." Other sections are equally revealing and emphasize Moderna's unwavering goal of advancing mRNA technologies that facilitate the future development of gene-therapy medicines for myriad applications.
Highlighting Moderna's nearly ten-year obsession with mRNA technology, the filing states that, in strategic alliance with the Defense Advanced Research Projects Agency (DARPA), Moderna (trade symbol MRNA) has been working on messenger RNA therapeutics since its "first modality"—prophylactic vaccines. A 2013 Moderna press release reveals the then three-year-old company received up to $25 million from DARPA to expand the "revolutionary new treatment modality" of mRNA products. The SEC filing clarifies that "modality" refers to technologies it believes "could enable a new group of potential mRNA medicines with shared product features." Currently, Moderna has seven modalities, which include mRNA-based cancer vaccines. The company explains its potential advantages in these areas, stating:
"Our potential advantages in these areas include: (1) mRNA can produce hard-to-make or complex proteins, (2) mRNA can replace defective genes, and (3) LNP (lipid nano-particle) delivery allows for repeat dosing."
 The variety of proteins made from mRNA within Moderna's development pipeline.
The variety of proteins made from mRNA within Moderna's development pipeline.
Moderna's mRNA-Focused Product Pipeline
Since its first program in 2014, Moderna and its strategic alliances—including with AstraZeneca, Merck, Vertex, and government-sponsored organizations and private foundations, including BARDA, DARPA, the National Institutes of Health (NIH), and the Bill & Melinda Gates Foundation—have "advanced in parallel a diverse development pipeline" which presently consists of 44 mRNA development programs across the company's 41 development candidates. Twenty-five candidates have entered the clinical phase, and one development candidate is subject to an open investigational new drug application (IND).

Along with the company's COVID-19 gene-therapy "vaccine," Moderna has seven other mRNA COVID-19 jabs for SARS-CoV-2 variants in various phases of development in its pipeline. Dubbed mRNA-1273, its COVID jab was co-developed with the National Institute of Allergy and Infectious Diseases, NIAID, and is also known as Spikevax, which to date has been injected into nearly 217 million humans in the U.S.
Similarly, the company is also working on a "next-generation" mRNA COVID-19 "vaccine," an "Endemic HCoV (human coronavirus) vaccine," and five mRNA flu "vaccines." Additionally, if all goes as planned, the company—which expects $21 billion in sales this year—hopes to push mRNA-based "COVID + Flu vaccine" and "COVID + Flu + Respiratory Syncytial Virus (RSV) vaccine" combo jabs. Moderna's vision for all of the mRNA products in the company's pipeline is for global distribution. The filing notes:
"We are also exploring agreements with governments around the world to establish local manufacturing capabilities in their countries, which would provide those governments with access to these annual [COVID] vaccines, as well as future pandemic preparedness capabilities."
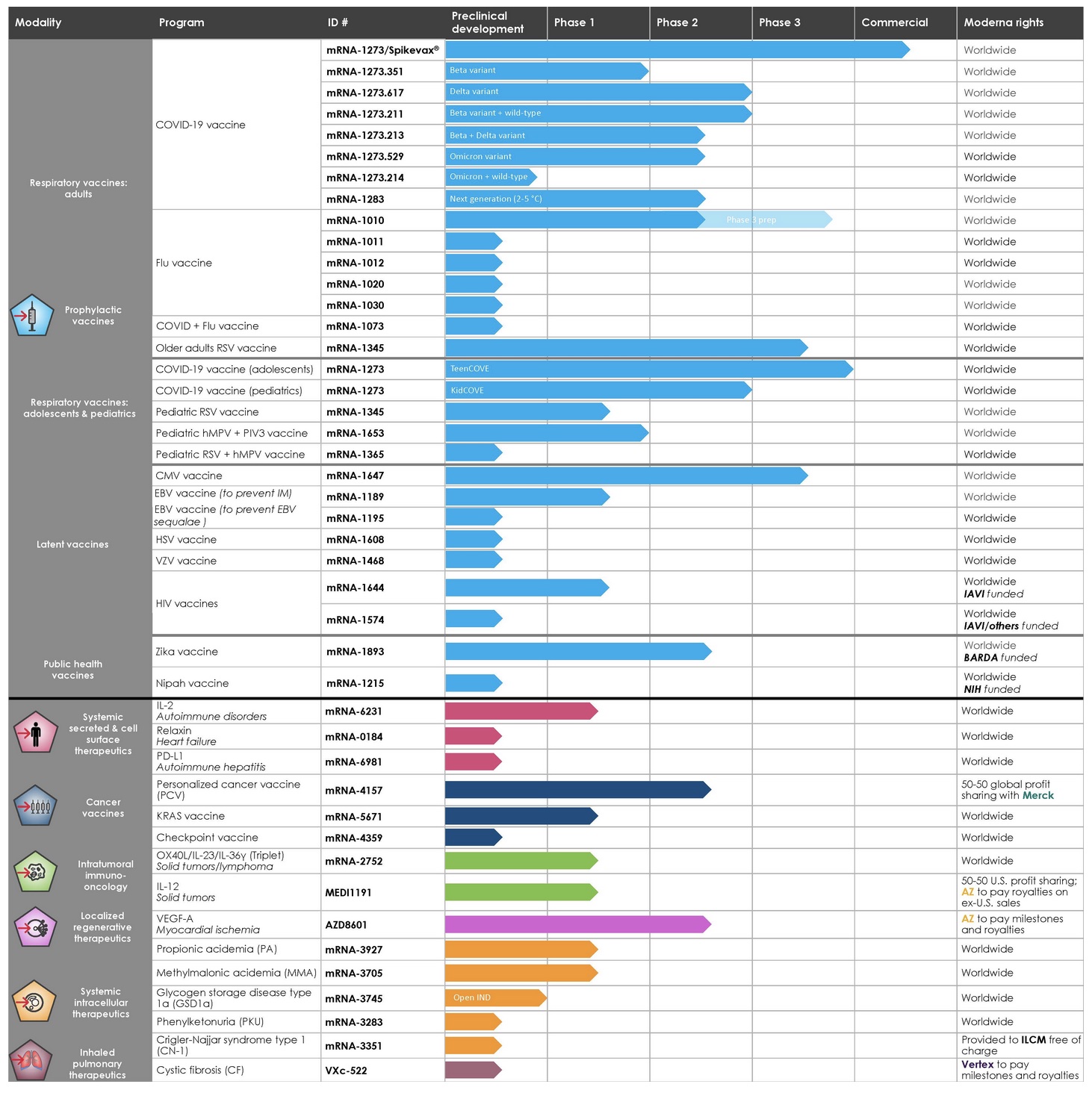 Moderna's full pipeline, grouped by seven mRNA modalities to date: 1) Prophylactic vaccines; 2) Systemic secreted and cell surface therapeutics; 3) Cancer vaccines; 4) Intratumoral immuno-oncology; 5) Localized regenerative therapeutics; 6) Systemic intracellular therapeutics; 7) Inhaled pulmonary therapeutics
Moderna's full pipeline, grouped by seven mRNA modalities to date: 1) Prophylactic vaccines; 2) Systemic secreted and cell surface therapeutics; 3) Cancer vaccines; 4) Intratumoral immuno-oncology; 5) Localized regenerative therapeutics; 6) Systemic intracellular therapeutics; 7) Inhaled pulmonary therapeutics
In the company's "prophylactic vaccine" modality, Moderna is actively working on 29 different vaccine programs based on mRNA technology. Seventeen programs are in clinical trials. The twenty-nine experimental products are divided into three categories—"vaccines against respiratory viruses" (including COVID-19), "vaccines against latent viruses," and the all-encompassing category of "other vaccines" (such as public health programs).
Moderna's "Third-Party Strategic Alliances"
Moderna's 10-K filing explains that to "accelerate the discovery and advancement of potential mRNA medicines across therapeutic areas, we have entered into, and intend to seek other opportunities to form alliances with a diverse group of strategic collaborators."
These partnerships include biotechnology and pharmaceutical companies, academic labs, foundations, research institutes, and government agencies. The company states it seeks to advance its discovery and development programs through collaborations with others while "leveraging our platform and our research and early development capabilities," including in the areas of gene-editing and cell therapy, where Moderna expects to bargain with its core mRNA and LNP capabilities and expand the reach of its technology.
Strategic Pharmaceutical Alliances
Moderna's pharmaceutical alliances include relationships with AstraZeneca for mRNA heart and cancer medicines, Merck for mRNA-based infectious diseases and cancer vaccines, and Vertex for potential mRNA and LNP medicines for the treatment of cystic fibrosis (CF). Vertex agreed to pay Moderna $75 million upfront to collaborate on gene-editing CF therapies.
As a side note, Merck—which invested $100 million in cash stock in Moderna in 2015 and then $125 million in 2018—has a lucrative $1.2 billion contract with the U.S. government to provide 1,696,629 courses of its EUA investigational drug molnupiravir. According to the partially redacted contract awarded by the Army, the price per treatment is $712.
Strategic Biotechnology Alliances
Armed with momentum and capital from the COVID-19 pandemic, Moderna—with 2,700 full-time employees, double from one year prior—wasted no time aligning its mRNA modalities with biotechnology companies. In Sept. 2020, the company teamed up with Chiesi to work on the discovery and development of mRNA therapeutics for treating pulmonary arterial hypertension (PAH). According to studies, PAH patients experience a higher mortality rate when infected with SARS-CoV-2.
As the pandemic raged on, in Nov. 2021, Moderna announced a collaboration with Megagenomi, whose "next-generation CRISPR-based and other novel gene editing systems," combined with the Moderna's mRNA and LNP technologies, will accelerate the development of in vivo gene editing therapeutics.
Then in Jan. 2022, Moderna entered a strategic alliance with Carisma. According to Carisma, the two companies entered into a calculated collaboration agreement to discover, develop and commercialize in vivo engineered chimeric antigen receptor monocyte (CAR-M) therapeutics for cancer treatment.
Strategic Government Alliances
Moderna's strategic alliances with government organizations to advance its mRNA modalities are equally as significant as those with pharmaceutical and biotechnology companies. In addition to Moderna's previously mentioned 2013 contract with DARPA, in Sept. 2020, the company entered into an agreement with the defense agency for an award of up to $56 million to fund the development of a mobile manufacturing prototype leveraging its existing manufacturing technology that is "capable of rapidly producing vaccines and therapeutics." As of Dec. 31, 2021, the committed funding net of revenue earned was $2 million, with an additional $42 million available under Agreement No. HR0011-20-9-0118 if DARPA exercises additional contract options.
Likewise, since 2016, Moderna has received $117 million of a $126 million agreement with the Biomedical Advanced Research and Development Authority (BARDA)—with support from Bill Gates—to help fund its Zika vaccine program. BARDA is a component of the Office of the Assistant Secretary for Preparedness and Response (ASPR) within the U.S. Department of Health and Human Services (HHS). Then, in April 2020, Moderna entered into another agreement with BARDA for an award of up to $483 million to accelerate the development of its COVID-19 "vaccine."
Interestingly, UNC scientist Dr. Ralph Baric (involved in controversial gain-of-function research) also helped develop Moderna's COVID jab and other treatments for COVID-19 like Remdesivir, which the FDA just approved for infants and young children. Hoping to include infants in its current COVID-19 "vaccine" experiment, in July 2020, Moderna amended its agreement with BARDA to include an additional commitment of up to $472 million to support the late-stage clinical development of its jab, adding an extra $144 million to support pediatric clinical trials for its mRNA shot. The SEC filing states:
"The maximum award from BARDA, inclusive of the 2020 and 2021 amendments, is $1.4 billion. Under the terms of the agreement, BARDA will fund the advancement of mRNA-1273 to FDA licensure. All contract options have been exercised. As of December 31, 2021, the remaining available funding net of revenue earned was $189 million."
Robert F. Kennedy Jr. Explains Why Big Pharma Wants Kids to get COVID Jab
Strategic Foundation Alliances
Finally, Moderna's collaboration with research institutes and foundations includes the Institute for Life Change Medicines (ILCM) and The Bill and Melinda Gates Foundation. In Sept. 2021, Moderna entered into an agreement with ILCM to develop a new mRNA therapeutic for Crigler-Najjar Type I (CN-1). The Filing reports ILCM received no upfront fees or downstream payments from Moderna and will be responsible for the clinical development of the new drug.
Collaborating on mRNA schemes for more than five years, Moderna and The Bill and Melinda Gates Foundation entered into a "global health project framework agreement" in Jan. 2016 to advance mRNA-based development projects for various infectious diseases. Gates committed up to $20 million in grant funding to support Moderna's initial project linked to the evaluation of antibody combinations in a preclinical setting as well as the conduct of a first-in-human Phase 1 clinical trial of a potential mRNA medicine to help prevent HIV infections.
Notably, Moderna's CEO Stéphane Bancel and Bill Gates were two of the "health, medicine, and biopharma leaders" attending the World Economic Forum's (WEF) annual meeting in Jan. 2019, along with WHO Director-General Tedros Adhanom Ghebreyesus. President Trump, Frances Collins, Mike Pompeo, Steve Mnuchin and other U.S. leaders canceled their trips. The pre-pandemic meeting focused on progress in vaccine development and financial investments in global health. With that target in mind, global health experts discussed appropriate actions to confront new biological threats not yet been identified, which they called "Disease X." Elaborating on the Gates Foundation's continuing relationship with Moderna, the filing states:
"Follow-on projects, which could bring total potential funding under the framework agreement up to $100 million (including the HIV antibody project) to support the development of additional mRNA-based projects for various infectious diseases, can be proposed and approved until the sixth anniversary of the framework agreement, subject to the terms of the framework agreement, including our obligation to grant to the Bill & Melinda Gates Foundation certain non-exclusive licenses."
Vaccinating Into a Pandemic is Always the Wrong Answer: Dr. Mike Yeadon
RISK FACTORS
With over 170 issued or allowed U.S. patents or applications (some related to COVID-19 and facing lawsuits) and more than 110 granted or allowed patents outside of the U.S. (and over 430 additional patents pending), Moderna assumes no accountability for the risk factors associated with its products. This lack of liability includes its FDA fast-tracked mRNA-based COVID-19 "vaccine," which the filing declares is Moderna's only commercial product and source of product revenues.
The filing explains that mRNA drug development faces many obstacles, including substantial clinical and regulatory risks, as well as the "negative perception" of the "efficacy, safety, or tolerability of any investigational medicines." Moreover, if labeled as "gene therapy" by the FDA, mRNA drugs may be subject to additional regulatory requirements.
Undoubtedly, the question of whether it is possible to safely and efficiently get genetic material (polynucleotides) into the nucleus of the majority of cells in the human body to halt illness (for COVID-19, this experimentation is being done via Moderna's synthetic mRNA spike protein) or so that any genetic defect (or transhuman genetic improvements) can be made leaves many experts, including Dr. Robert Malone, shouting "stop." Importantly, Malone insists that these synthetic mRNA-like molecules may account for many unusual effects and "striking adverse events" associated with this new class of "vaccines." He explains:
"This is why there is so much concern about the possibility that the mRNA-like polynucleotides used in the 'RNA vaccines' may travel into the nucleus (where the DNA chromosomes reside) and insert or recombine with a cellular genome after reverse transcription (RNA->DNA). Normally, with DNA-based gene therapy technologies, the FDA requires genotoxicity studies for this reason, but the FDA did not treat the 'mRNA vaccine" technology as a gene therapy product'."
Dr. Robert Malone: Synthetic mRNA Cannot Be Removed From The Body
With the recent reporting of Moderna recalling thousands of COVID jabs in Europe due to contamination, the company explains it faces "intense competition" concerning its COVID-19 jab, and its "vaccine" may not "continue to compete favorably with existing or future products."
Indeed, the emergency use authorization (EUA) of Moderna and Pfizer's "vaccines" rushed them into the market and was based on the FDA's determination that there were no existing effective treatments for COVID. Still, Moderna's filing points out that competitor vaccines, or other treatments, may prove to be safer, more effective, more convenient, have fewer side effects, be easier to ship or distribute, or be developed at a lower cost than its vaccine. Ivermectin and Hydroxychloroquine, which government experts and legacy news outlets immediately censored, are two such treatments that come to mind. The filing explains:
"The actual or perceived success or failure of other entities may adversely impact our ability to commercialize our COVID-19 vaccine. We also will face competition from products that have already been approved and accepted by the medical community for the treatment of conditions we target."
In addition to safety and efficacy concerns, the exhaustive list of risk factors associated with Moderna's mRNA "investigational products" covers everything from production flaws to potential bribery, cyberattacks, and climate change. However, many experts insist the most considerable risk is the ongoing mRNA COVID-19 gene therapy experiment itself.
Thanks to DARPA, the FDA, NIH, the World Economic Forum (WEF), and the World Health Organization (WHO), the current COVID-19 "vaccine" experimentation on humanity—which Moderna hopes will soon include infants and young children—undeniably lays the foundation for The Great Reset. Many argue that the goal is to advance mRNA technology while people fear COVID-19. Keenly aware of the uncertainty and the possibility of its collapse, Moderna states in the filing:
"Certain features in our development candidates and investigational medicines, including those related to mRNA, chemical modifications, surface chemistries, LNPs, and their components, may result in risks that apply to some or all of our programs and modalities. As our development candidates and investigational medicines progress, we or others may determine that:
certain of our risk allocation decisions were incorrect or insufficient;
we made platform-level technology mistakes;
individual programs or our mRNA science in general has technology or biology risks that were unknown or under-appreciated;
our choices on how to develop our infrastructure to support our scale will result in an inability to manufacture our investigational medicines for clinical trials or otherwise impair our manufacturing;
or we have allocated resources in such a way that we cannot recover large investments or rapidly re-direct capital.
As we progress our programs through clinical development, new technical challenges may arise that cause an entire modality to fail. Additionally, any portfolio spanning risks, whether known or unknown, if realized in any one of our programs, would have a material and adverse effect on our other programs and on our business as a whole."


