As the U.S. Food and Drug Administration (FDA) hints at a future model for developing new COVID-19 "vaccines" similar to the approach taken toward annual flu-like vaccines, a new study published Tuesday by the New England Journal of Medicine reveals that a fourth Pfizer/BioNTech experimental COVID-19 jab offers little protection against infection. The study, which analyzed 1.25 million vaccinated individuals 60 years or older in Israel, occurred from Jan. 1 through Mar. 2, 2022, when the omicron variant of SARS-CoV-2 was predominant and indicated protection from a fourth booster wanes quickly.
The analysis comes as both the FDA and the Centers for Disease Control and Prevention signed off late last month to expand its emergency use authorization (EUA) to allow additional booster doses as early as four months after their first booster (of Pfizer or Moderna) for all Americans 50 and older, along with those as young as 12 who are also immunocompromised due to certain medical conditions. Nevertheless, Dr. Peter Marks, the FDA's director of its Center for Biological Evaluation and Research, said in a meeting a week after the approval of the fourth shot:
"There is a certain diminishing return by giving the same vaccine over and over. We have had enough evolution of this virus that it would make sense to want to try to cover some of the genetic diversity that has been introduced into the mix.
By this fall, we may be on to a new variant. It could be sigma. It may be tau. There may be something new that may be circulating that we'll have to deal with. We're going to have to make a good guess at what may be most effective."
MkQM_MrxmHtmK-r4xLb0tg
FDA, CDC, and NIH Meet Wednesday to Discuss Boosters
In an apparent stroke of good timing, the FDA advisory committee, the CDC, and members of the National Institute of Health (NIH) are meeting today to discuss considerations "for future COVID-19 booster doses and the process for selecting specific strains of the SARS-CoV-2 virus for COVID-19 vaccines to address current and emerging variants." Speaking of the Apr. 6 meeting (event materials here), Marks declared:
"As we prepare for future needs to address COVID-19, prevention in the form of vaccines remains our best defense against the disease and any potentially severe consequences. Now is the time to discuss the need for future boosters as we aim to move forward safely, with COVID-19 becoming a virus like others such as influenza that we prepare for, protect against, and treat. Bringing together our panel of expert scientific external advisors in an open, transparent discussion about booster vaccination is an important step to gain insight, input, and expert advice as we begin to formulate the best regulatory strategy to address COVID-19 and virus variants going forward."
I_VMp1Jc8ppSpcCXbVZm7w
Meanwhile, using the Israeli Ministry of Health database, the ten study authors in the NEJM study discovered that the rate of infection and cases of severe COVID-19 were lower after a fourth dose of the "vaccine" than after only three doses. However, the "protection against confirmed infection appeared short-lived, whereas protection against severe illness did not wane during the study period," they added. Still, as reported by CNN, the short six-week study period wasn't long enough to determine precisely how long this protection against severe illness lasts.
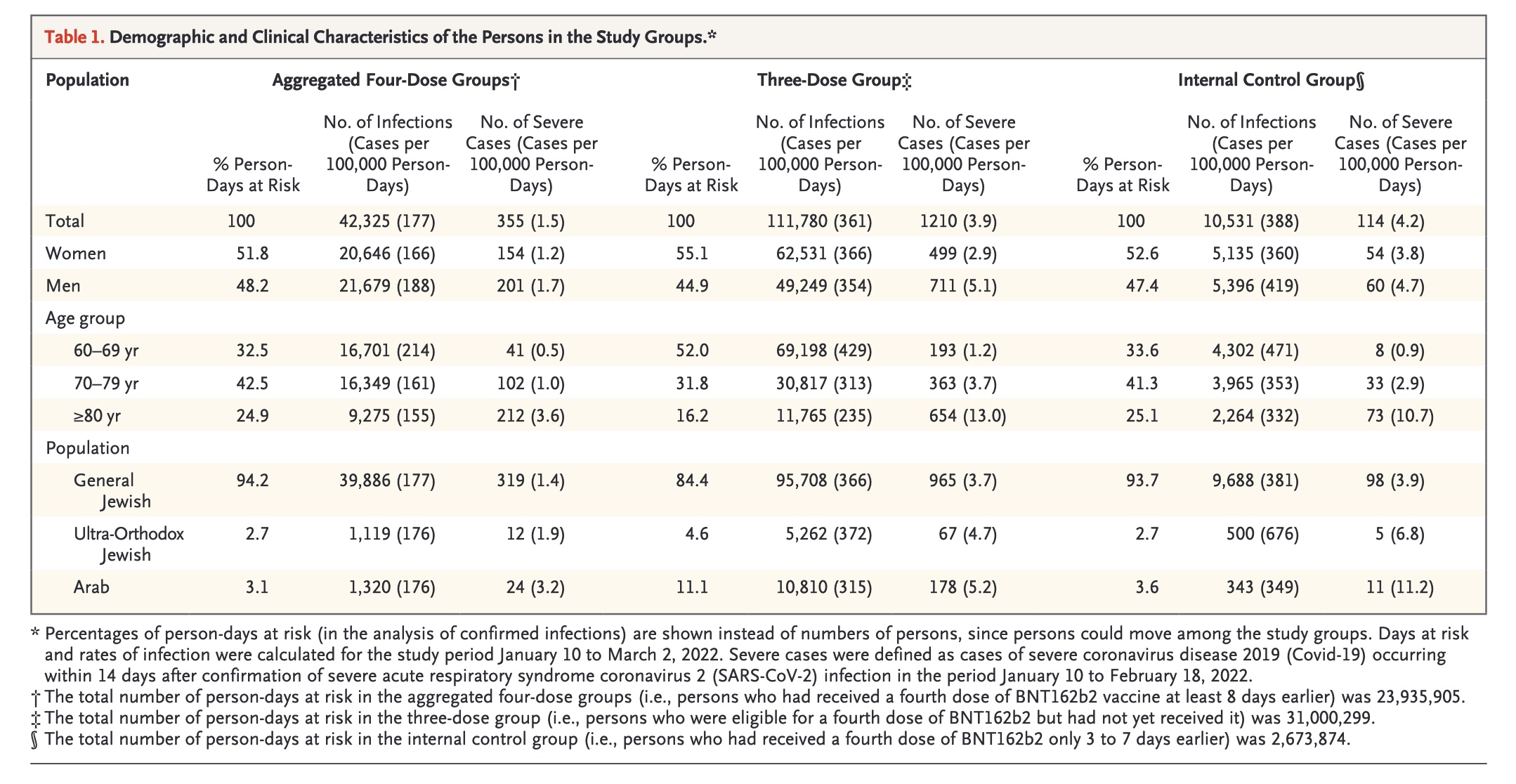 Screenshot / NEJM Protection by a Fourth Dose of BNT162b2 against Omicron in Israel
Screenshot / NEJM Protection by a Fourth Dose of BNT162b2 against Omicron in Israel
Israel's COVID Data Sharing Agreement with Pfizer
Additionally, the research only focused on potential protection following a third and fourth dose and did not include unvaccinated individuals who have natural immunity following recovery from COVID-19 for comparison. It also did not add to the discussion on whether people under 60 years of age may need a fourth dose. Previous research in Israel indicated that a fourth dose had little impact on younger healthy populations in terms of protection from COVID-19 infection. Interestingly, the oversight section of the study pointed out that the Israeli Ministry and Health and Pfizer "have a data-sharing agreement," adding, "only the final results of this study were shared."
Indeed, in Jan. 2021, Israel beat out larger countries on COVID-19 inoculations by entering the agreement with Pfizer, which secured the country a steady stream of the pharma giant's clinical trial-phase mRNA COVID-19 product vials and allowed them to jab a larger share of its citizens than other nations. Israel shared a redacted copy of the agreement after repeated calls for transparency and issues over data security and privacy, with some expressing concerns that the details of the agreement may not be shared for thirty years. As pointed out by NPR, "Israel paid a premium, locked in an early supply of Pfizer-BioNTech vaccines, and struck a unique deal: vaccines for data."
I_VMp1Jc8ppSpcCXbVZm7w
According to the agreement, the nation of nearly 9 million promised Pfizer a speedy vaccine rollout, along with data from Israel's centralized trove of medical statistics to examine "whether herd immunity is achieved after reaching a certain percentage of vaccination coverage in Israel." Israel's health minister Yuli Edelstein noted at the time, "We said to Pfizer ... that the moment they give us the vaccine, we'll be able to vaccinate at the speed they've never heard of."
While it is not clear whether today's FDA hearing will include the NEJM's Israeli study on Pfizer's boosters or whether its results will be used to push for annual COVID-19 shots, experts in Israel have expressed concern over the nation's agreement with Pfizer for over a year. The Israel Democracy Institute's Tehilla Shwartz Altshuler, a data privacy advocate and a leading voice questioning the Pfizer data deal, voiced his concerns last January, asserting:
"We need to understand that [Israel's agreement with Pfizer] is going to be one of the, I would say, widest medical experiments on humans at the 21st century."
Dr. Makary—Authorities don't study natural immunity because results would undermine vax push.


