A year ago, in an article titled "Vaccine Expert Warns: We Made a Big Mistake," UncoverDC highlighted an ominous warning from Canadian vaccine expert Dr. Byram Bridle. As the vaccine rollout got underway, the viral immunologist cautioned that serious side effects could occur in those who receive an mRNA-based COVID-19 "vaccine." Bridle's alert came in May 2021, after he and international colleagues examined a biodistribution study submitted by Pfizer to the Japanese health regulatory agency. Dr. Bridle, who was personally attacked in a very public campaign to discredit him after he spoke up last May, is once again speaking up to declare that a moratorium on mRNA "vaccines" is needed.
Specifically—as Bridle previously warned—the lipid nanoparticle (LNP) delivery system in the mRNA-based shots spread far and wide throughout the body. This fact directly contradicts the initial public health messaging that "mRNA jabs behave like traditional vaccines." In an in-depth April 21 Substack post, Bridle notes that the mRNA "vaccine" safety statements repeated by health officials caused him to, at the time of their unveiling, assume the LNPs in the mRNA jabs had somehow been modified to stay at the injection site, which was news to him. Clearly, that is not the case, and according to Bridle, highlights one of the first rules of thumb when practicing science:
"Transparently presented raw and/or peer-reviewed data are the cornerstones of objective science; not personal proclamations of data disseminated via media releases."
LNPs Were Designed for Systemic Distribution
As reiterated by Bridle, unlike traditional vaccine technology, the systemic distribution of lipid nanoparticles was a necessary component of LNP technology due to its need to attempt to replace genes in cells throughout the body, including the brain in order to treat things like Alzheimer's disease and Parkinson's disease. Likewise, the systemic distribution was necessary to deliver chemotherapeutic drugs to cancers that could have spread anywhere in the body, including the brain. Explaining the basis for LNP technology further, Bridle revealed:
"Also, this wide distribution is accomplished by helping the LNPs avoid uptake by phagocytic cells, which are the cells of the immune system that promote induction of immune responses! I postulate that the mRNA inoculations would function better as vaccines if mRNAs could be stabilized without PEG [polyethylene glycol]."
Bridle's recent post discussed the biodistribution of LNPs, referencing a more detailed English version of the Japanese study he analyzed last year. The more comprehensive study (released by the FDA), conducted on Wistar Han Rats, tracked the distribution of LNPs manufactured by British Columbia-based Acuitas Therapeutics. As described by Bridle, LNPs are essentially very tiny bubbles of fat used to deliver genetic material into our cells. Pointing out Pfizer's use of LNPs in its COVID-19 jab, Bridle notes:
"In the case of Pfizer's "vaccine," the payload is a messenger RNA molecule that encodes the spike protein from SARS-CoV-2, which is the causative agent of COVID-19. When the mRNA gets into a cell, it uses the existing manufacturing capacity of the cell to make copies of the spike protein."
Cancers Taking Off "Like Wildfire" - Dr. Ryan Cole Explains How the Shots Disrupt the Immune System
Pfizer Biodistribution Study on LNPs Failed to Include Spike Protein
Unfortunately, as stressed by Bridle, Pfizer was "never required to perform the same biodistribution study with the same 'vaccine' formulation that is being used in people." Rather than analyzing the spike protein, the study targeted LNPs, which carried an mRNA encoding protein that can be used in imaging studies. Therefore, Bridle said the expression of the spike protein was not evaluated "so we can not assess where the protein ends up in the body, only where the LNPs went."
Outlining the foundation of Pfizer's insufficient study, Bridle explained that rats are a generally used animal for pre-clinical research. In this case, three males and three females were euthanized at each of multiple time points to harvest a variety of tissues to quantify the amount of LNPs in them. As explained by Bridle, the incomplete study replaced the spike protein with a surrogate protein called luciferase, a light-producing enzyme naturally found in fireflies. He added:
"The mRNA in this study encoded 'luciferase,' a protein that can be used to visualize where the mRNA is being converted into proteins. However, this analysis was not done in this particular experiment. "[3H]-labeled" means the LNPs were tritiated or tagged with tritium. Tritium is a radioactive form of hydrogen. This allowed the LNPs to be quantified in tissues by measuring radioactivity. "ALC-0315" is a fat-based molecule that helps to compact mRNA into a nanoparticle and also promotes the introduction of the mRNA into a cell. "ALC-0159 "is a molecule that contains polyethylene glycol (PEG). You might have heard a lot about PEG in the context of COVID-19' vaccines' since it is the component that has been most associated with causing anaphylactic shock (a severe hyperacute allergic reaction) in some recipients. It serves a couple of functions:
1. This is the commonly mentioned purpose: It helps to stabilize the mRNA molecules; if mRNA degrades before it gets into a cell, the protein that the immune system is supposed to target will never be produced.
2. This is the other function of PEG that you may never have heard about (but those who have followed mRNA vaccine technologies have known about for many years): It helps the LNPs avoid the immune system to promote wide-spread dissemination throughout the body. Yes, you read that correctly."
oSb47bjK6_1c07N6RPzhqQ
The systemic distribution of LNPs is "not anywhere close" to the behavior of traditional vaccines. Indeed, the wide distribution is achieved by helping the LNPs avoid uptake by phagocytic cells of the immune system, which promotes the induction of immune responses. Again, according to Bridle, mRNA inoculations would perform better as vaccines if mRNAs could be stabilized without PEG.
Overview of the Acuitas-Sponsored Pfizer LNP Rat Study
In his April article, Dr. Bridle thoroughly examined Pfizer's report of the biodistribution study sponsored by Acuitas, titled "A Tissue Distribution Study of a [3H]-Labelled Lipid Nanoparticle-mRNA Formulation Containing ALC-0315 and ALC-0159 Following Intramuscular Administration in Wistar Han Rats." The study's first attempt was a failure due to overt toxicity. Bridle asserted that "on the basis of this failed first attempt and an unwillingness to adjust course, regulatory agencies never should have let Pfizer proceed with their vaccine until a large array of safety questions were addressed experimentally."
The study included significant failures and limitations. For example, urine and fecal samples were collected but never analyzed. Bridle commented that this is a shame considering the real-world debate about the possible shedding of mRNA "vaccine" components and/or the spike protein they encode. Additionally, some samples were analyzed fresh, while others were frozen for an unknown period and then thawed for analysis. Once thawed, the stability of mRNAs was compromised and may explain why mRNA and the spike proteins were not evaluated in the study. This approach could have influenced conditions for LNPs as well.
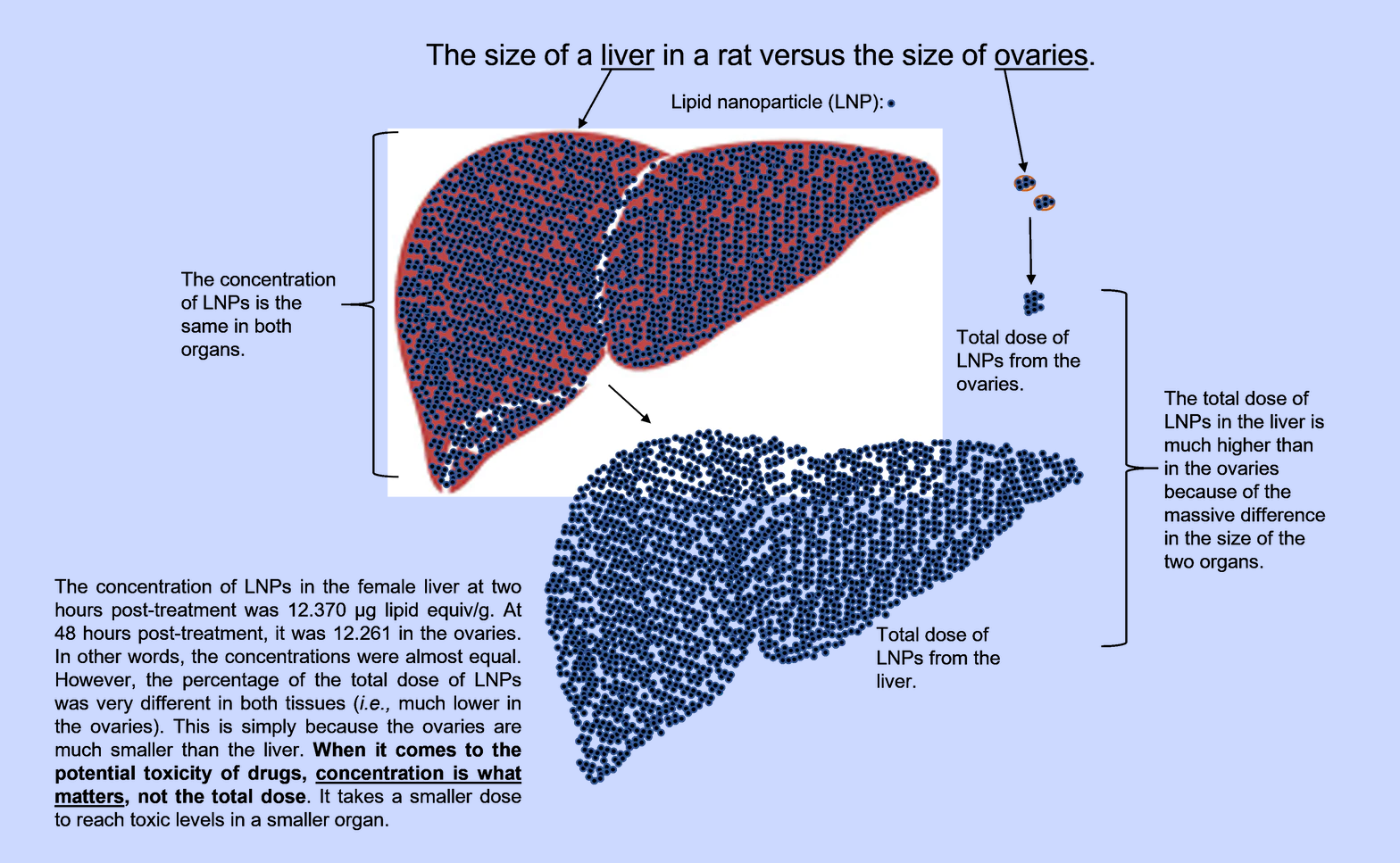
In studying the biodistribution data, Bridle explained that, unlike the Japanese study which hid important sex differences, the FDA-released study separates the data for males and females. Despite the study's shortcomings and the adjustment to dosing following the extreme toxicity of the study's first attempt, Bridle noted that the clinical observations reveal "something disturbing." He wrote:
"The mRNA "vaccine" proved to be acutely toxic to one of the three females that was monitored beyond 24-hours post-injection. No obvious signs of illness were observed with the three males that were allowed to live past 24 hours (the endpoint was 48 hours). Some may say it was only one female rat, but it is 1/3 (33.3%) of the female rats.
Further, it is unknown whether others would have become visibly sick had the observation period been extended beyond 48 hours; especially when one understands that concentrations of LNPs were still rising in many female tissues (discussed below).
Remember, in Canada, AstraZeneca's COVID-19' vaccine' was deemed to be too unsafe for adults when public statements suggested it was causing dangerous blood clots in 1:55,000 people. So, writing off a 1:3 incidence of obvious toxicity in the pre-clinical study was unwise."
 Screenshot / Covid Chronicles / Dr. Byram Bridle: A Moratorium on mRNA 'Vaccines' is Needed; Re-Visiting the Biodistribution of Lipid Nanoparticles
Screenshot / Covid Chronicles / Dr. Byram Bridle: A Moratorium on mRNA 'Vaccines' is Needed; Re-Visiting the Biodistribution of Lipid Nanoparticles
Biodistribution is Everywhere
Bridle remarked that page 20 of the report states, "The overall injection site concentrations and % dose values were higher in males than in females. Since concentrations in other tissues were broadly similar between the sexes, it is likely that the higher injection site values in males were a result of its more consistent identification and collection in males." However, Bridle importantly pointed out flaws in that assessment, declaring:
"This fails to account for the fact that concentrations in tissues tended to peak or plateau at relatively early time points in males and were still climbing at the last time point in females. It also doesn't consider the possibility that the LNPs might have been accumulating at higher concentrations than males in tissues that were not evaluated in the study."
Similarly, Bridle emphasized that it is a "long-accepted" scientific fact that LNPs used to deliver the mRNA in "vaccines" can be toxic. This fact was openly discussed with the media before the COVID-19 pandemic and is precisely why some big pharmaceutical companies strategically focused on using them as vaccine technologies instead of for gene therapies and to deliver drugs. Bridle added:
"A good quality vaccine, such as those used in the mandated childhood series, only require one or two doses for a person's lifetime. It was assumed the same would hold true for mRNA vaccines. Repeated administration of lipid nanoparticles, especially over a limited period of time, is known to be toxic."
Meanwhile, although this article does not encompass all of Bridle's excellent substack post, his comprehensive assessment of the Pfizer study released by the FDA underscores the sheer magnitude and scope of the inaccurate messaging delivered to the public about mRNA "vaccines" and lipid nanoparticles. Government experts, including those at the CDC and FDA, repeatedly stated that most of the mRNA COVID-19 dose remains at the injection site. They made this declaration despite possessing the Pfizer study at hand, which revealed that a "mere 15-minutes post-injection," only 7 percent of the mRNA jab could be detected at the injection site. Speaking of this startling reality, Bridle exclaimed:
"In females, as little as 7% of the injected dose remained at the site of inoculation. This means the vast majority of it went somewhere else. Where did it go? The short answer is everywhere."


