A critical new study in Nature Immunology titled "Children develop robust and sustained cross-reactive spike-specific immune responses to SARS-CoV-2 infections" reveals children build up strong and durable immunity when they have been infected with COVID-19. Considerably stable beyond 12 months, these immune response findings "provide insight into the relative clinical protection that occurs in most children." Significantly, the study states:
"We showed that children display a characteristically robust and sustained adaptive immune response against SARS-CoV-2 with substantial cross-reactivity against other hCoVs. Spike-specific T cell responses were more than twice as high in children. And broadly stable beyond 12 months. This is likely to contribute to the relative clinical protection that occurs in most children."
As of December 30, 2021, nearly 285 million people have been infected with SARS-CoV-2 worldwide, of which around 5.4 million have died. SARS-CoV-2 infection in children is typically asymptomatic or mild and contrasts with high rates of hospitalizations and death in older adults. As such, the variation in how age affects an individual's response has sparked an ongoing interest in "understanding the profile of the immune response to SARS-CoV-2 in children." Repeatedly, research confirms that "children diagnosed with COVID-19 have an overall excellent prognosis."
The study, partly funded by UK Research and Innovation (UKRI)/National Institute for Health Research through the UK Coronavirus Immunology Consortium (P.M.), points out that one possible explanation for the different immune responses to COVID-19 across various age groups might be "the timing of exposure to the four additional endemic human coronaviruses (hCoVs)." These comprise the Beta-coronaviruses OC43 and HKU-1, which have 38% and 35% amino acid homology with SARS-CoV-2, and the more distantly related Alpha-coronaviruses NL63 and 229E, each with around 31% homology.
These four coronaviruses (hCoVs) cause routine mild childhood infections, and antibody seroconversion occurs typically before age 5. The study notes that infection with one of the Alpha- or Beta-coronaviruses provides short-term immunity against reinfection from coronaviruses and represents brief cross-reactive immunity within the subtypes. As such, "recent hCoV infection might presensitize children against SARS-CoV-2 infection and may explain cross-reactive SARS-CoV-2-neutralizing antibodies in some seronegative children." With that in mind, the researchers compared antibody levels against the four hCoVs in children and adults who were seronegative and seropositive for SARS-CoV-2.
The study compared antibody and cellular immunity in children aged 3-11 and adults aged 20 to 71. Blood samples were obtained from 91 children and 154 adults, including 35 children and 81 adults who tested positive for the virus in previous rounds of testing. All infections were asymptomatic or mild, and no staff or students in the cohort required medical care or hospitalization.
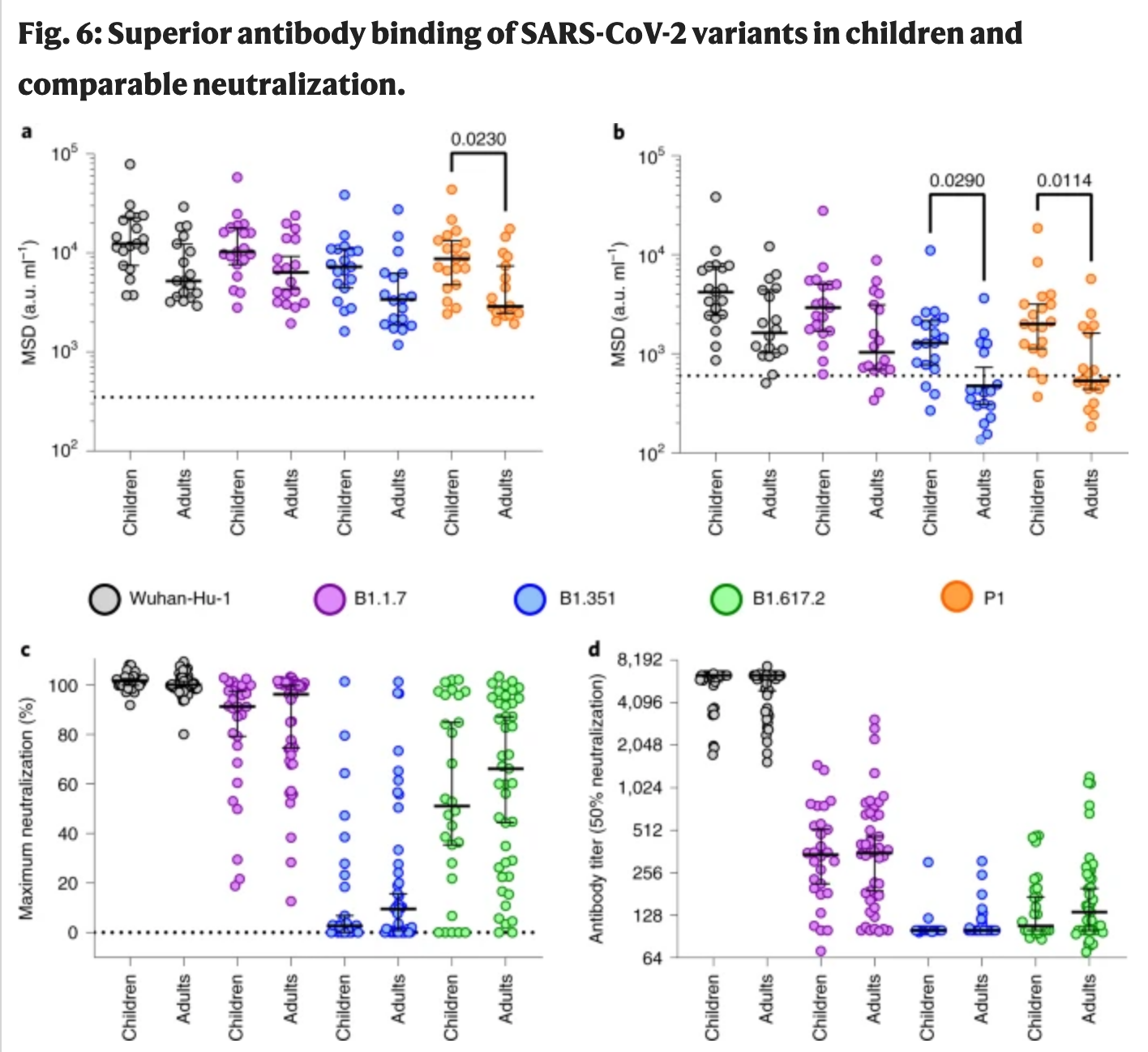 a,b, Antibody binding to spike (a) and RBD proteins (b) from SARS-CoV-2 variants using plasma from children (n = 19) or adults (n = 18). The bars indicate the geometric mean ± 95% CI. Kruskal–Wallis with Dunn's multiple comparisons tests were used. c,d, Live virus neutralization assays on SARS-CoV-2 variants displayed as maximal neutralization of infection (c) and titer at 50% neutralization (d) using plasma from children (n = 28) or adults (n = 43). The bars indicate the median ± 95% CI.
a,b, Antibody binding to spike (a) and RBD proteins (b) from SARS-CoV-2 variants using plasma from children (n = 19) or adults (n = 18). The bars indicate the geometric mean ± 95% CI. Kruskal–Wallis with Dunn's multiple comparisons tests were used. c,d, Live virus neutralization assays on SARS-CoV-2 variants displayed as maximal neutralization of infection (c) and titer at 50% neutralization (d) using plasma from children (n = 28) or adults (n = 43). The bars indicate the median ± 95% CI.
Antibody responses to viral proteins were broadly identical in seropositive children and adults. However, the study revealed that geometric mean antibody titers against all four areas (hCoVs) were more significant in children, with the N-terminal domain (NTD) and receptor-binding domain (RBD) showing 2.3-fold and 1.7-fold increases, respectively, though these did not achieve statistical significance. Unlike earlier findings, the authors found antibody responses to nucleoprotein, with a 1.3-fold higher antibody titer in children than in adults.
Compared to the seronegative group, SARS-CoV-2 seropositive adults had a 1.2–1.4-fold rise in hCoV titers. In contrast, SARS-CoV-2 seropositive children had significantly greater antibody levels against all four viruses, with 2.3, 1.9, 1.5, and 2.1-fold higher antibody levels than the seronegative group. Notably, seropositive children had similar levels of hCoV-specific antibodies to adults, whereas seronegative children had lower responses than adults.
The authors also examined antibody titers against influenza subtypes and respiratory syncytial virus in relation to SARS-CoV-2 serostatus to see if this outcome was exclusive to hCoV or a more general effect of SARS-CoV-2 infection on antibody responses against heterologous infection. No change in antibody titers against these viruses was observed in children or adults. These findings indicate that SARS-CoV-2 infection improves antibody responses against hCoVs in children.
Following the increase in hCoV-specific antibody titers in children after SARS-CoV-2 infection, the authors analyzed whether this was cross-reactive with SARS-CoV-2 or an hCoV-specific response. Accordingly, plasma samples were pre-absorbed with recombinant S1 or S2 domain protein from SARS-CoV-2 before measuring antibody levels to both SARS-CoV-2 and the four hCoV subtypes.
The researchers observed that antibody titers against the whole spike protein were remarkably reduced after pre-absorption with both the S1 and S2 domains. RBD- and NTD-specific antibodies against SARS-CoV-2 were absorbed by the S1 domain but not the S2 domain, with no effect on the nucleocapsid-specific binding for either domain.
The S1 domain did not affect antibody binding to any of the four hCoV subtypes, suggesting that cross-reactive antibodies against this domain are doubtful. Comparatively, the S2 domain inhibited antibody binding to OC43 and HKU-1. Regarding the alpha coronaviruses NL63 and 229e, no such effect was seen.
The study joins a long list of peer-reviewed research demonstrating that children exhibit a characteristically robust and sustained adaptive immune response against SARS-CoV-2 with significant cross-reactivity against other hCoVs. Undoubtedly, this natural immunity contributes to the relative clinical protection acquired by children.


