The prominent British Medical Journal (BMJ)—a weekly peer-reviewed trade journal in the United Kingdom—published an article this week titled, "COVID-19 vaccines and treatments; we must have raw data, now." Presented by senior editor Peter Doshi, the BMJ asserts data from vaccine and therapeutics manufacturers "should be fully and immediately available for public scrutiny."
Undoubtedly, big pharma cannot disregard the importance of promptly sharing clinical trial data. Still, at the moment, the BMJ points out they are repeating the same mistakes made ten years ago with the Tamiflu fiasco, which "heralded a decade of unprecedented attention" to the significance of sharing drug trial data and ultimately promised a new era in data transparency.
Nonetheless, we continue to lack crucial data from drug manufacturers supplying the "accepted" treatments for COVID-19 (Moderna, Pfizer, AstraZeneca for "vaccines" as well as Regeneron and Gilead Sciences for therapeutics, to name a few). Stating that "the errors of the last pandemic are being repeated," the journal reminded:
"In the pages of The BMJ a decade ago, in the middle of a different pandemic, it came to light that governments around the world had spent billions stockpiling antivirals for influenza that had not been shown to reduce the risk of complications, hospital admissions, or death. The majority of trials that underpinned regulatory approval and government stockpiling of oseltamivir (Tamiflu) were sponsored by the manufacturer; most were unpublished, those that were published were ghostwritten by writers paid by the manufacturer, the people listed as principal authors lacked access to the raw data, and academics who requested access to the data for independent analysis were denied.
The errors of the last pandemic are being repeated. Memories are short. Today, despite the global rollout of COVID-19 vaccines and treatments, the anonymized participant-level data underlying the trials for these new products remain inaccessible to doctors, researchers, and the public—and are likely to remain that way for years to come. This is morally indefensible for all trials, but especially for those involving major public health interventions."
Historically, access to data for drugs and vaccines has been relatively limited to journal article publications, hard-to-access, and challenging to read regulatory reports. Yet, critical appraisal of clinical trials is essential in decision-making on all levels and cannot be credibly performed on journal publications alone. Doshi, an associate professor of pharmaceutical health services research at the University of Maryland School of Pharmacy, criticized medical journals for not holding pharmaceutical companies accountable. He also denounced government regulators in the U.S. Food and Drug Administration (FDA) for "being complicit in data secrecy." He wrote:
"Journal editors, systematic reviewers, and the writers of clinical practice guideline[s] generally obtain little beyond a journal publication, but regulatory agencies receive far more granular data as part of the regulatory review process."
Unlike the FDA, the European Medicine Agency (EMA) and Health Canada have publicly initiated policies and platforms to facilitate data access. The EMA's former executive director and the senior medical officer explained that "relying solely on the publications of clinical trials in scientific journals as the basis of healthcare decisions is not a good idea... Drug regulators have been aware of this limitation for a long time and routinely obtain and access the full documentation (rather than just publications)."
Despite receiving more raw data on trials than almost any other agency, the FDA refuses to proactively release the vital information. As reported by UncoverDC, following a freedom of information (FOIA) request for Pfizer vaccine data, the FDA offered to release 500 pages a month. In court, the agency argued that "publicly releasing data was slow owing [to] the need to first redact sensitive information." However, this month, a judge rejected the FDA's offer and demanded the data be released at a pace of 55,000 pages a month. The data are to be made public on the website Public Health and Medical Professionals for Transparency (phmpt.org), the party requesting the documents.
Twelve years ago, the BMJ called for the immediate release of raw data from clinical trials. They reiterate that same call now, insisting that transparent decision-making is essential, claiming, "Regulators and public health bodies could release details, such as why vaccine trials were not designed to test efficacy against infection and spread of SARS-CoV-2." According to numerous experts brave enough to speak up, including Doshi, had that outcome been known, countries would have understood sooner about the impact of vaccines on transmission and been able to plan accordingly.
Indeed, as the global vaccine rollout continues, it is unjustifiable and not in the best interests of patients and the public that we are left to trust "in the system," with the distant hope that urgent underlying data "may become available for independent scrutiny at some point in the future." Without question, transparency is the key to building trust. Doshi explains it is an essential pathway to answering people's honest questions about the safety and efficacy of vaccines and other treatments and the clinical and public health policies established for their use. Summarizing the call for transparency held by a growing number of experts and citizens alike seeking answers from government agencies and drug manufacturers, the article shines a spotlight on a large part of the concern, stating:
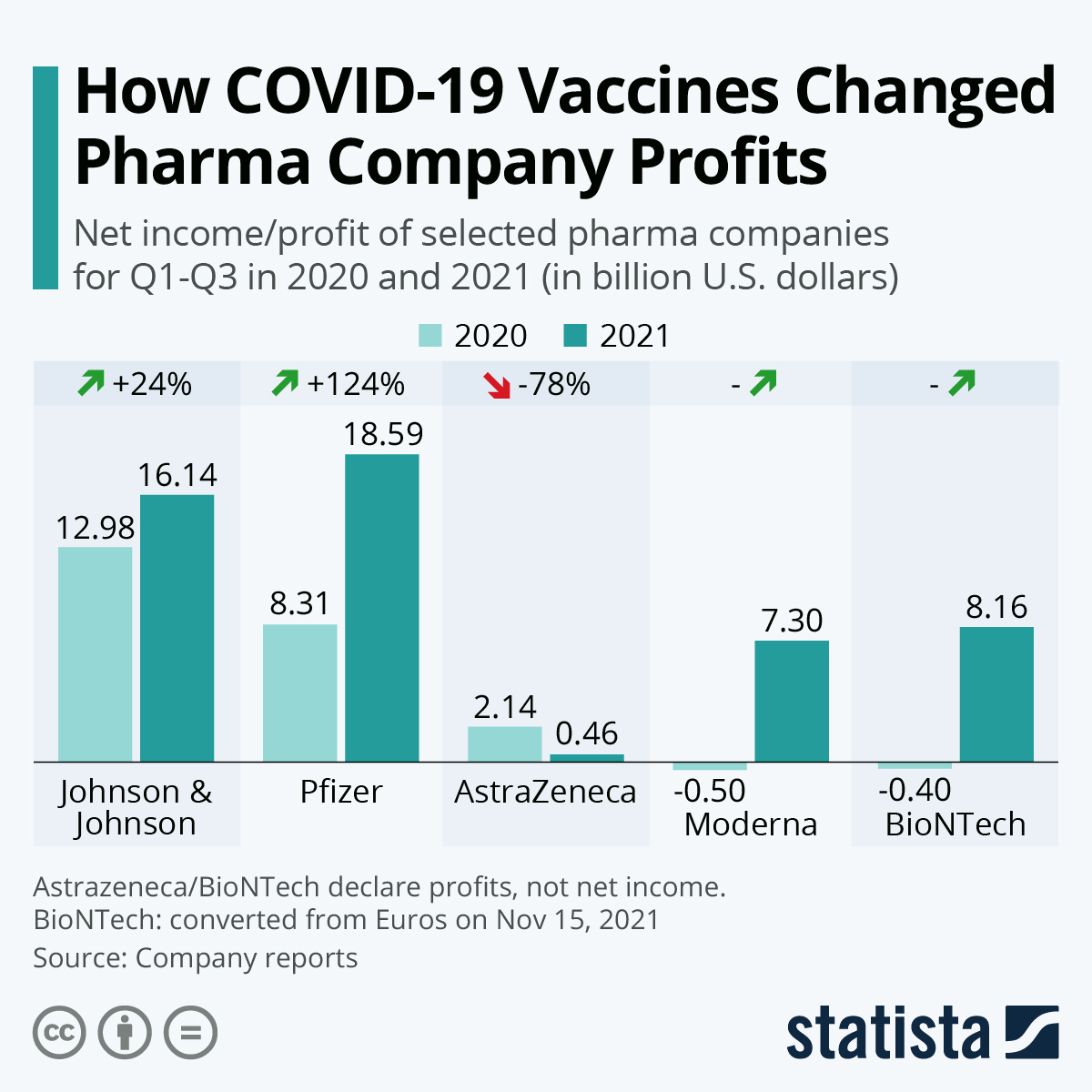
"Big pharma is the least trusted industry. At least three of the many companies making COVID-19 vaccines have past criminal and civil settlements costing them billions of dollars. One pleaded guilty to fraud. Other companies have no pre-COVID track record. Now the COVID pandemic has minted many new pharma billionaires, and vaccine manufacturers have reported tens of billions in revenue.
Pharmaceutical companies are reaping vast profits without adequate independent scrutiny of their scientific claims. The purpose of regulators is not to dance to the tune of rich global corporations and enrich them further; it is to protect the health of their populations. We need complete data transparency for all studies, we need it in the public interest, and we need it now."